Overcoming The Challenge Of Dienes In The Cracked Naphtha

Controlling dienes in cracked naphtha is critical to meet strict gasoline specs and keep FCC units running efficiently. This article explores cutting-edge hydroprocessing technologies that help refiners stay profitable and compliant.
One of the biggest challenges to the crude oil refining industry in the last decades was the development of technologies capable of reducing the environmental impact of the derivatives while raising the performance of these compounds.
The hydro-processing technologies allow the production of cleaner and better performance derivatives while simultaneously making possible the recovery of higher yields of added value products from bottom barrel streams in the crude oil refining.
The value addition to naphtha streams is especially hard in some cases, mainly to the streams produced by deep conversion processes like Fluid Catalytic Cracking (FCC).
Despite the current trend of reducing transportation fuel demand, some markets still present significant dependence on these crude oil derivatives to sustain economic development; this is especially true in developing economies like Brazil.
The production of high-quality gasoline is still fundamental to crude oil refiners aiming to meet the market demand. Considering the current specifications, the synergy between Fluid Catalytic Cracking units (FCC) and Cracked Naphtha Hydrodesulphurization is fundamental to allow the refiners to reach profitable operations, mainly in markets with high demand transportation fuels.
Gasoline Production Process
The final gasoline is composed of a blending of different naphtha streams, as presented in Figure 1.
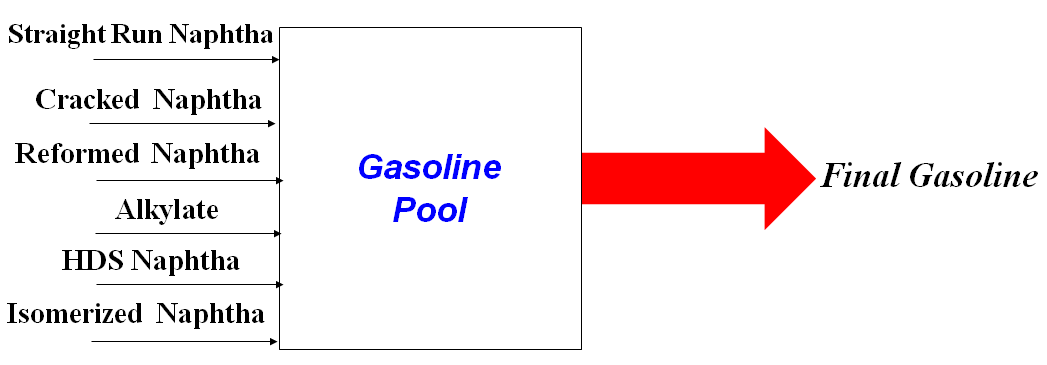
The use of straight run and reformed naphtha is usually minimized, aiming to direct these streams to the petrochemical intermediates market due to the higher added value of these streams.
Cracked naphtha (Naphtha from FCC) contributes positively to the octane number of the final gasoline; however, due to the current restrictions related to the sulfur content in the gasoline (maximum of 10 ppm), the use of cracked naphtha without treatment step is limited.
Refineries with catalytic alkylation units in their refining scheme usually direct this stream to produce aviation gasoline, which has a higher market value when compared with automotive gasoline. For this reason, the participation of the alkylation naphtha is minimized in the composition of this fuel.
Isomerization naphtha has low contaminants content (sulfur and nitrogen) and high-octane number. For these reasons, the participation of this stream in the formulation of gasoline is maximized in refineries that have isomerization units in the refining scheme.
Refiners can add butanes to the gasoline pool in markets with high gasoline demand. However, participation is limited due to this stream's high vapor pressure, which can lead to breaking quality requirements (Reid Vapor Pressure – RVP). Usually, butanes are added to the LPG pool, respecting the limits to avoid damaging the mixture quality related to heavy limitations.
Nowadays, due to the environmental and quality regulations over gasoline, the hydrodesulfurization of cracked naphtha is fundamental for the refiners to meet the sulfur content in the final gasoline that can be lower than 10 ppm in most restrictive markets.
FCC Fundamentals
The cracked naphtha is one of the most valuable streams produced in the FCC unit. Typically, the average yield in fluid catalytic cracking units is 55% in volume in cracked naphtha and 30% in LPG. Figure 2 presents a scheme for the main fractionator of the FCC unit with the principal product streams.
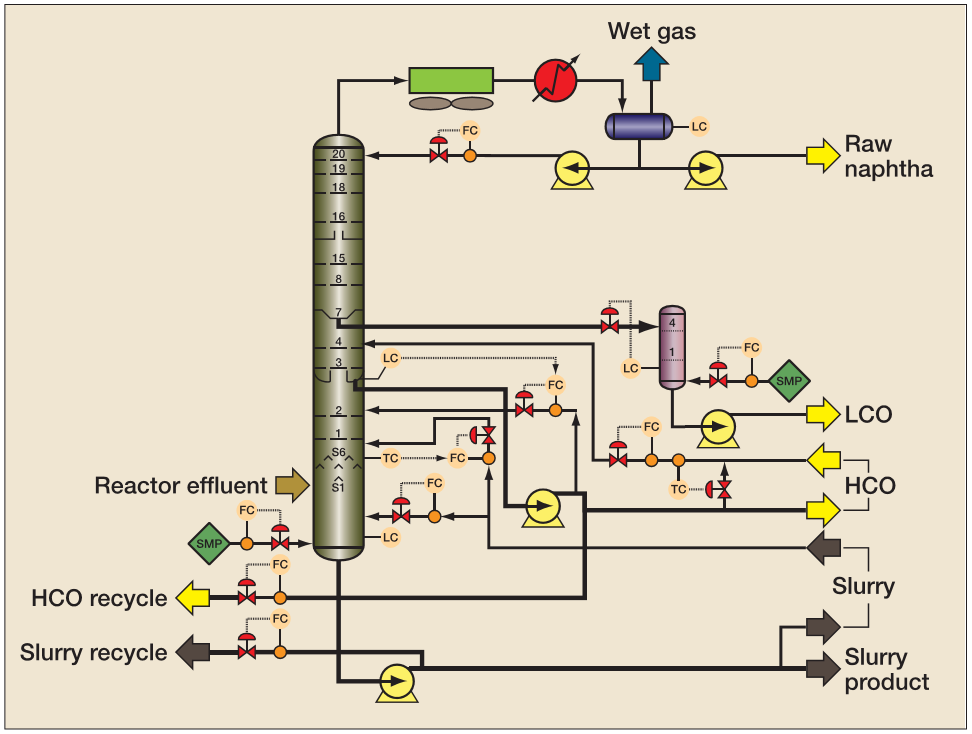
The overhead products from the main fractionator are still in the gaseous phase and are sent to the gas separation section. After treatment, the fuel gas is sent to the refinery fuel gas ring to remove H2S, where it is burned in fired heaters, while the LPG is directed to treatment (MEROX) and further commercialization.
The LPG produced by the FCC unit has a high content of light olefins (mainly propylene). In some crude oil refineries, the LPG stream is processed in a propylene separation unit to recover the propylene that has a higher added value than LPG.
Cracked naphtha is usually sent to a refinery gasoline pool formed by naphtha produced by other process units like straight run naphtha, naphtha from the catalytic reforming unit, etc.
Due to the production process (deep conversion of residues), the cracked naphtha has high sulfur content. To attend to the current environmental legislation, this stream needs to be processed to reduce the content of the contaminants, mainly sulfur.
The cracked naphtha sulfur removal represents a great refining challenge. It is necessary to remove the sulfur components without molecules saturation that gives high octane number for gasoline (mainly olefins).
FCC Naphtha Hydrotreating Technologies
The technical challenge makes it hard to treat the naphtha from the Fluid Catalytic Cracking Unit (FCC) dedicated to producing gasoline, one of the most consumed crude derivatives in the world market.
The use of the crude oil, increasingly heavier and consequently with higher contaminants content, mainly sulfur, further increased the pressure of the regulatory agents upon the refiners to reduce the contaminants contained in the derivatives, primarily diesel and gasoline.
Hydrotreating is the primary technology applied to reduce the contaminants contained in the crude oil derivatives. Figure 3 shows a simplified process scheme for a typical low severity hydrotreating unit.
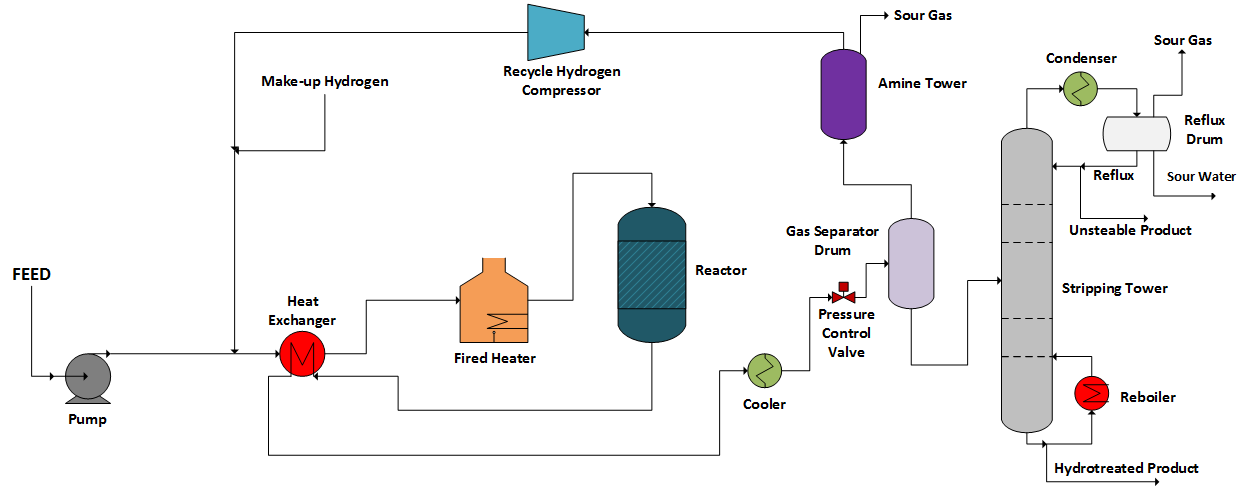
The process scheme presented in Figure 3 is standard and widely applied in the crude oil refining industry. However, to produce high-quality gasoline, the use is limited once hydrotreating reactions fatally lead to olefins saturation, which is responsible for the high-octane number in the final gasoline.
Straight run naphtha is usually directed to conventional hydrotreating, as presented in Figure 3. It has a reduced olefin content at the same time, which presents reduced sulfur content. In this case, the mild hydrotreating process is effective.
On the other hand, naphtha from FCC tends to show high sulfur concentration and high olefin content, the processing of this stream in conventional hydrotreating units would be less effective.
The main chemical reactions associated with cracked naphtha hydrotreating can be represented as follows:
R-CH=CH2 + H2 → R-CH2-CH3 (Olefins Saturation) (1)
R-SH + H2 → R-H + H2S (Hydrodesulfurization) (2)
Where R is a hydrocarbon.
As aforementioned, in the case of naphtha hydrotreating to gasoline, the objective is to minimize the reaction (1) and maximize the yield of the reaction (2), aiming to keep the high-octane number while the sulfur content is reduced, making the naphtha more environment friendly.
Hydro Processing Technologies
Over the last decades, technology process licensors have devoted their efforts to developing hydro processing technologies capable of achieving these objectives.
Selective Hydrotreating Methods
1. Select Fining™ Technology
UOP Company commercializes the Select Fining™ technology that applies fixed bed reactors to promote selective hydrotreating of naphtha. This leads to the unstable compounds (diolefins) saturation in the first reactor and the heavier olefins (higher sulfur content) saturation in the second reactor using a suitable catalyst.
2. PRIME G+™ Process
Nowadays, one of the most applied technologies to reduce the sulfur content in the cracked naphtha is the PRIME G+™ process, developed by the Axens Company.
The process uses the concept of which the tendency of the sulfur to concentrate in the heavier fractions of the cracked naphtha. Therefore, feed stream fractionation before the hydrotreating step is conducted, as presented in Figure 4.
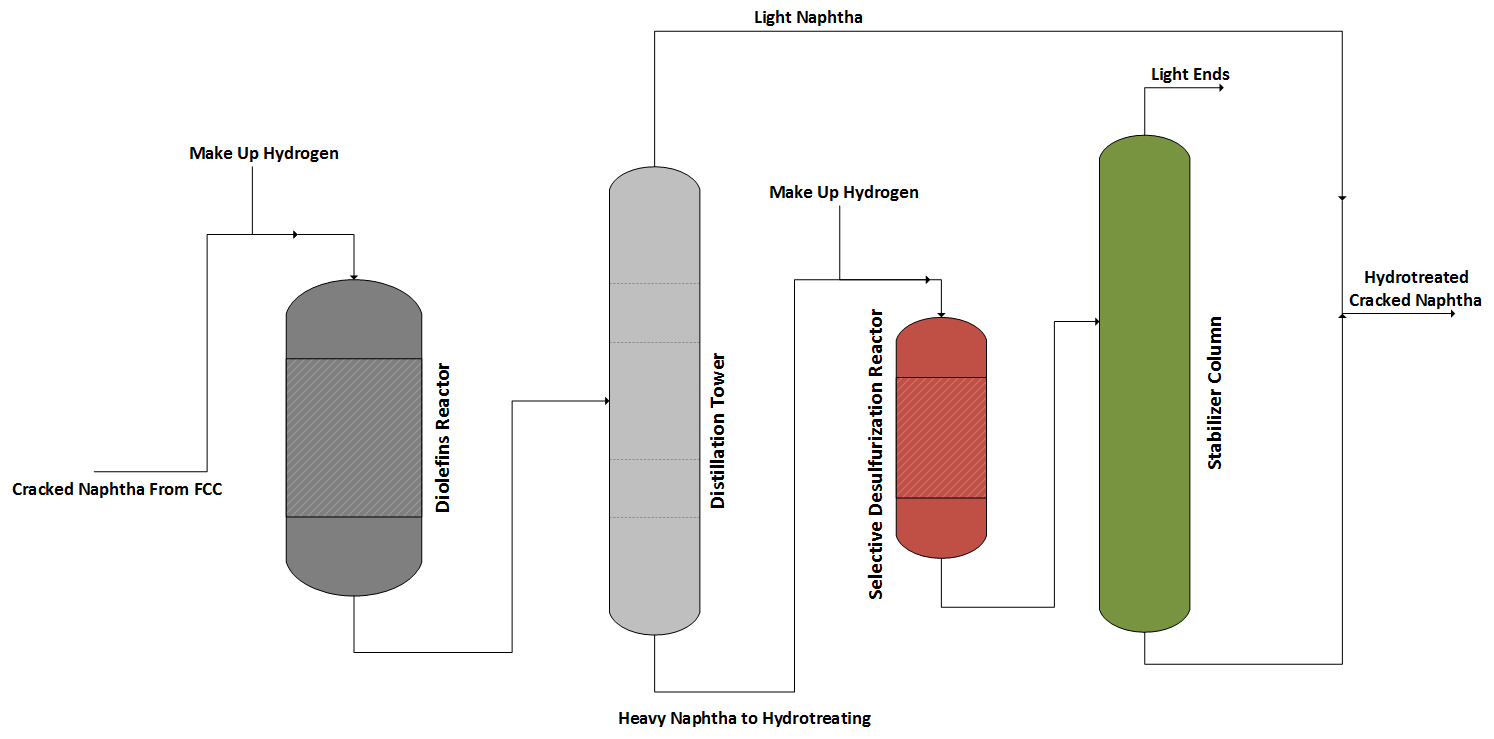
The feed stream is fed to a hydrotreating reactor to promote the diolefins saturation, and then the stream is separated into light and heavy fractions in a distillation tower. While the light naphtha is recovered in the top, the heavy fraction is removed from the bottom of the column and sent to a selective hydrotreating section leading to a minimum octane loss.
The hydrotreated naphtha is separated in a stabilizer column to remove light compounds in the sequence. The bottom product is mixed with the light fraction, and the final product is directed to the refinery gasoline pool.
3. Scan finning™ Process
Another technology that applies selective hydrotreating to reduce the sulfur content in the cracked naphtha is the Scan finning™ process developed by ExxonMobil Company.
In this case, fixed bed reactors are applied without feed stream fractionation. Regarding the catalysts dedicated to selective hydrogenation of FCC naphtha, we can highlight the product HyOctane™ developed by the Haldor Topsoe company.
4. GT-DeSulph™ Process
The GT-DeSulph™ process developed by Sulzer-GTC Engineering Company applies the extractive distillation principle associated with selective hydrotreating. The use of aromatic solvents allows the removal of thiophene compounds from cracked naphtha.
Non-Selective Hydrotreating Methods
1. ISAL™ Technology
Another interesting route adopted by the licensors is the non-selective hydrotreating followed by an octane index recovery step, the ISAL™ technology by UOP Company employs this principle. In this case, through a suitable catalyst, olefin saturation occurs, and posteriorly the molecular rearrangement is conducted to recover the octane index lost in the first step.
2. S-Zorb™ Process
The S-Zorb™ process developed by ConocoPhillips applies the concept of chemical adsorption of the sulfur compounds on zinc oxide (ZnO). It has a continuous regeneration system of the adsorbent through the controlled burn of the adsorbed sulfur compounds.
3. CD Hydro + CDHDS™ Process
Another attractive technology applied to FCC naphtha desulfurization is the CD Hydro + CDHDS™ process commercialized by the Lummus company, based on reactive distillation and hydrodesulfurization, as presented in Figure 5.
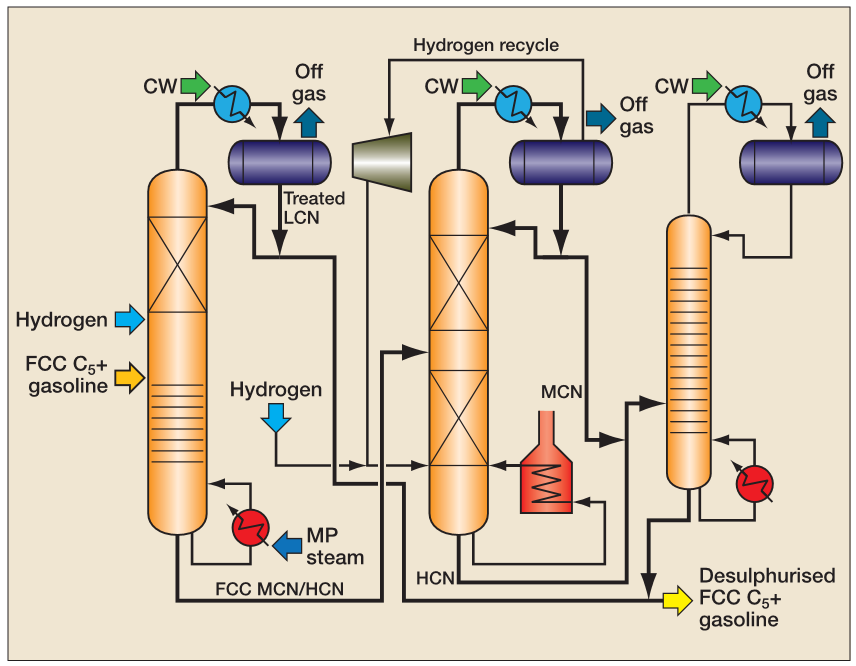
FCC naphtha is a fundamental stream for the refiners to meet the automotive gasoline requirements (minimum octane number) and the adequate treatment of this process stream is necessary to ensure adherence to environmental regulations and raise the refiner's profitability once the cracked naphtha yield in the fluid catalytic cracking units can reach over 50%.
It is important to remember that the main objective of the Selective Hydrotreating Unit (SHU) is to control the contaminant’s content in the hydrodesulfurization section. Especially polymers and gum precursors like diolefins tend to produce coke in the catalyst pores, leading to quick deactivation and pressure drop in the catalytic bed.
A Special Challenge – Dienes (Diolefins) Control
A key parameter in the cracked naphtha is the conjugated diene content; these compounds are chemically unstable and tend to suffer oxidation reactions leading to gum formation in the reactors in hydrodesulfurization units, leading to high-pressure drop and lower operation campaigns.
The dienes formation in fluid catalytic cracking units occurs mainly in the transfer line between the reaction section and the fractionator. This phenomenon is caused by the absence of catalyst in this section and, due to the elevated temperature, thermal cracking occurs, favoring the dienes production.
Due to these characteristics, the crude oil refiners usually control the dienes production through the quench injection at some point post riser aiming to reduce the temperature, avoiding then thermal cracking and dienes formation. The literature quotes that reducing close to 50% of the dienes formation is possible by applying a post riser quench with light cycle oil (LCO).
The main process licensors recommend the quench injection in the separator vessel (diluted phase). Once the injection in the transfer line can lead to hydrocarbon condensation and coke deposition in the bottom of the main fractionator.
The use of quench in the riser tends to raise the production of fuel gas and coke, which can limit the FCC units in the machines like blower and cold area compressors. Figure 6 presents a process flow diagram for the R2R™ FCC technology commercialized by Axens company.
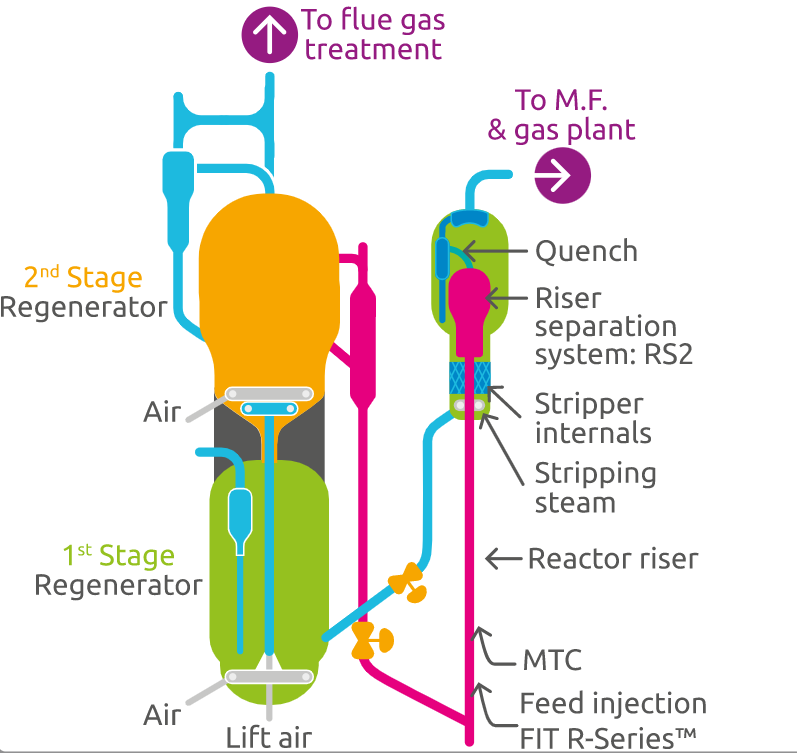
The quench injection in the post riser section is even more important to FCC units operating under high severity. The operating conditions favor the formation of dienes at higher rates (higher temperature in the post- riser sections).
The dienes concentration in the cracked naphtha is usually controlled through the Maleic Anhydride Value (MAV), which is defined by a quantity of Maleic Anhydride (in mg) necessary to react with one gram of cracked naphtha through the Diels-Alder mechanism as presented in Figure 7.
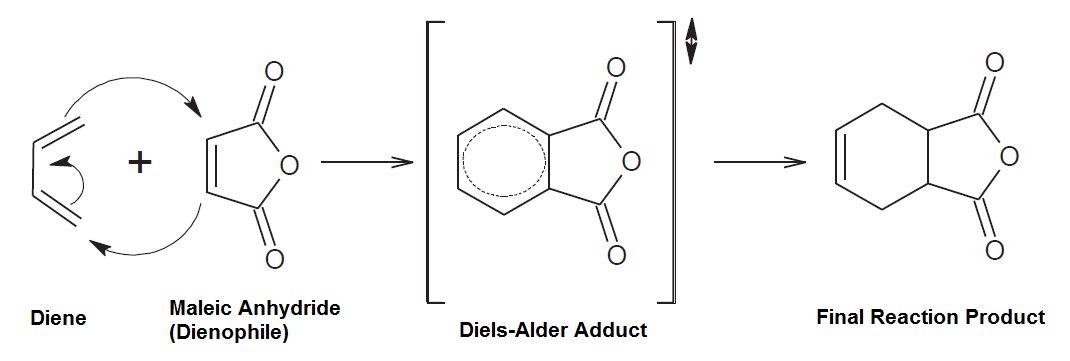
Some refiners usually control the MAV below 4,0 mg/g, but it is important to consider that the dienes concentration is not the unique parameter to keep a reliable operation in the cracked naphtha hydrodesulfurization unit.
Control of the concentration of light compounds in the feed (C4) is essential to avoid sudden vaporization in the catalyst bed, leading to lower reaction temperatures in the selective hydrogenation unit that cause lower conversion, consequently raising the diolefins (dienes) concentration in the primary reaction section that can lead to gum formation, reducing the operational lifecycle.
The light concentration in the cracked naphtha is controlled through the Reid Vapor Pressure; another side effect of the diolefins concentration in FCC naphtha hydrotreaters is related to the polymerization in the catalyst bed leading to higher pressure drop and shorter operating lifecycle.
Face to the current specifications of gasoline, the cracked naphtha hydrodesulfurization unit became fundamental to allow refiners relying on FCC units in their refining scheme to produce marketable gasoline. An unplanned shutdown of this unit can quickly lead to an FCC shutdown and, in extreme cases, the interruption of refinery operations.
In some cases, premature activity loss can be observed in the Selective Hydrotreating Unit (SHU). This scenario can be overcome through lower temperature in the FCC unit (TRX), which can be compensated through a high activity of the FCC catalyst.
It is essential to consider that the matter with dienes in the cracked naphtha tends to raise with the operating severity of the fluid catalytic cracking unit. In the last few years, we have seen a trend among the refiners aiming to revamp their FCC units focusing on improving the olefins throughput, which can intensify the problems with dienes content in the cracked naphtha.
It is important to consider including a quench stream in the post riser section to control this parameter in the cracked naphtha and preserve the health of hydrodesulfurization reactors.
Controlling the Pressure Drop - Guard Beds against Contaminants
Control of the catalyst lifecycle is a key issue for refiners, especially in FCC naphtha hydrodesulfurization units. One of the main strategies adopted in the last few years is using guard beds in hydro-processing catalysts to protect the catalysts, ensuring a longer and more profitable operating campaign.
The main objective of the guard bed is to protect the primary and active catalyst against:
· Particulates from the feedstock can be dragged like sediments, catalysts powder, and corrosion products capable of producing physical fouling.
· Heavier hydrocarbons capable of leading to coking deposition
Chemically unstable hydrocarbons are capable of producing gum, like olefins and diolefins.
· Metals and catalysts poisons like Ni, V, Fe, Si, Na, etc.
Hydroprocessing Catalysts Technologies
1. STAX™ Technology
As aforementioned, due to the higher concentration of contaminants, the guard beds are most common in hydroprocessing units dedicated to processing heavier feedstocks, as quoted above.
Usually, a grading strategy in the catalyst bed aims to establish a staggering pore diameter and activity to the catalysts, keeping the catalysts in the top more resistant to the contaminants acting as a filter, protecting then the most active catalyst in the bottom section. Figure 8 presents an example of hydro processing catalysts grading according to STAX™ technology by Albemarle Company.
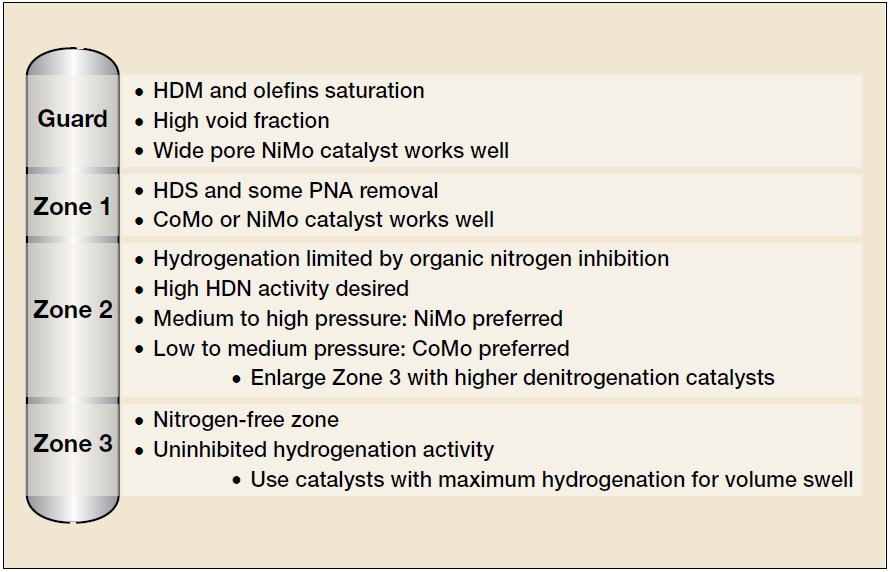
In Figure 8, the guard bed will be responsible for controlling the content of the contaminants (mainly metals) in the following catalyst sections as well as for reducing the carbon residue (CCR) and particulates concentration, keeping the activity and improving the lifecycle of the hydroprocessing unit.
2. CatTrap™ Technology
Among the most known catalyst protection technologies available in the market, we can quote the CatTrap™ technology developed by Crystaphase Company. This technology applies a ceramic bed acting as a filter to particulate materials, controlling especially the pressure drop in the catalyst bed.
Other Catalytic systems
For units dedicated to treating bottom barrel streams, the hydroprocessing catalyst needs present high activity and be resistant to the content of the high contaminants (sulfur, nitrogen, and silicon). Some companies have made efforts to develop catalytic systems that are capable of attending to these requirements.
As examples of these technologies, we can quote the START™ system by Advanced Refining Technologies (ART) Company, the UNITY™ system developed by UOP Company, and the SENTRY™ catalysts by Criterion Catalysts Company, and the TK-449 Silicon Trap™ by Haldor Topsoe Company.
Figure 9 presents a comparative study developed by Haldor Topsoe Company related to improving the cycle length of a naphtha hydrotreating unit by applying grading particles to control the content of the contaminants over the primary catalyst.
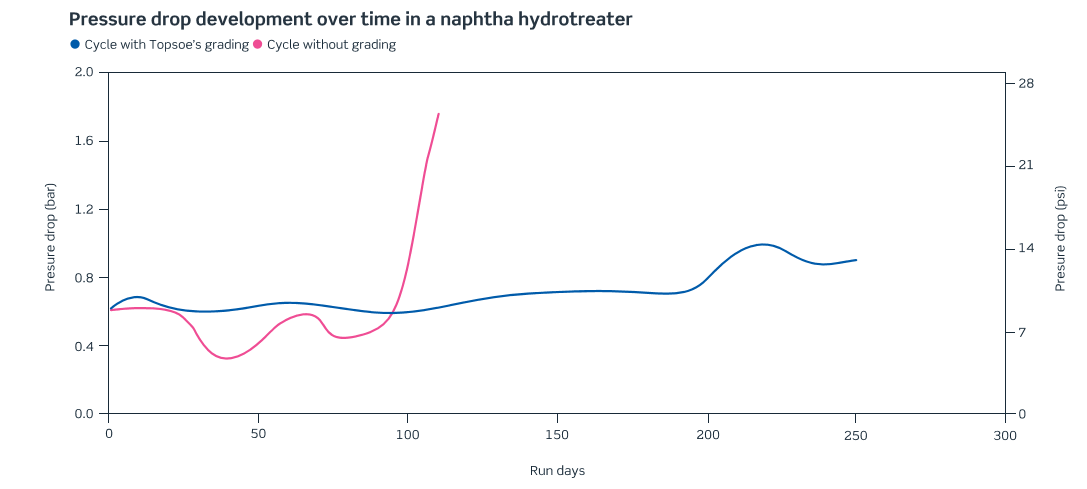
The increasing relevance of the hydro processing technologies to the downstream industry requires even more attention from refineries aiming to keep profitable and reliable operations in these units. The guard bed technologies have an important role in allowing the achievement of this goal. As presented in Figure 8 these technologies can improve in a significant manner the operational lifecycle of the hydroprocessing units.
Conclusion
Despite the recent forecasts, the transportation fuels demand responds by a significant part of revenues in the downstream market, especially in developing economies.
The capacity to add value to the naphtha from catalytic cracking processes like FCC is fundamental to ensure better profitability for refiners and minimize the environmental footprint in the crude oil processing chain.
As aforementioned, an integrated operation between FCC and Cracked Naphtha Hydrodesulphurization units is fundamental for the refiners to achieve profitably, reliable, and competitive operations in the downstream market.
As presented above, the dienes control in the FCC naphtha is a key topic that needs special attention from the refiners. According to the refining scheme, the cracked naphtha desulfurization unit's unavailability can make the operation of the whole refinery unfeasible.
This article was contributed by our expert Marcio Wagner da Silva
Frequently Asked Questions Answered by Marcio Wagner da Silva
Q1. What is the fluid catalytic cracking process?
The FCC process is a refining process applied to add value to heavy streams of crude oil after the distillation to maximize the yield of valuable derivatives like gasoline, LPG, and light olefins like propylene.
Q2. What is the importance of FCC and hydrocracker units in a refinery?
The main objective of these processing units is to improve the bottom barrel conversion capacity of the refining hardware converting the residue streams into more valuable derivatives and then raises the refining margins.
Q3. What is the purpose of hydrotreating?
The main objective of the hydrotreating units is to remove contaminants (Sulfur, Nitrogen, etc.) from the processing streams or final derivatives to minimize the environmental impacts of the fuels during the burning process (fewer emissions of NOx and SOx).
Comments
No comments yet. Be the first to comment!
